3-D maps of a protein show how it helps organs filter out toxic substances
Images of LRP2 in simulated cell environments reveal the structural changes that let it catch molecules outside a cell and release them inside.
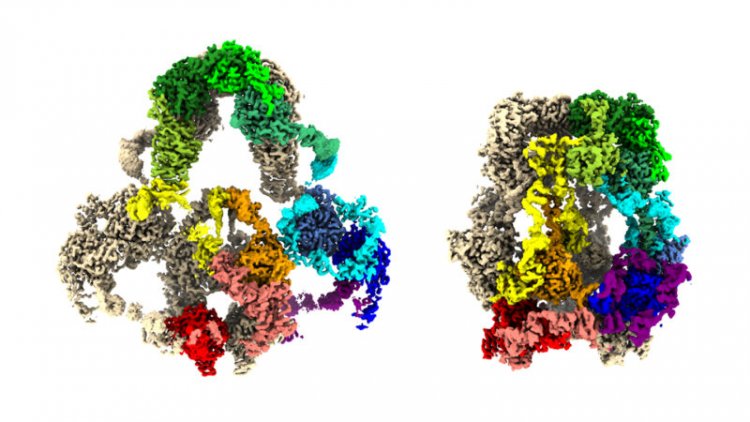
A close look at one protein shows how it moves molecular passengers into cells in the kidneys, brain and elsewhere.
The protein LRP2 is part of a delivery service, catching certain molecules outside a cell and ferrying them in. Now, 3-D maps of LRP2 reveal the protein’s structure and how it captures and releases molecules, researchers report February 6 in Cell. The protein adopts a more open shape, like a net, at the near-neutral pH outside cells. But in the acidic environment inside cells, the protein crumples to drop off any passengers.
The shape of LRP2’s structure — and how it enables so many functions — has stumped scientists for decades. The protein helps the kidneys and brain filter out toxic substances, and it operates in other places too, like the lungs and inner ears. When the protein doesn’t function properly, a host of health conditions can occur, including chronic kidney disease and Donnai-Barrow syndrome, a genetic disorder that affects the kidneys and brain.
The various conditions associated with LRP2 dysfunction come from the protein’s numerous responsibilities — it binds to more than 75 different molecules. That’s a huge amount for one protein, earning it the nickname “molecular flypaper,” says nephrologist Jonathan Barasch of Columbia University.
Typically, LRP2 sits at a cell membrane’s surface, waiting to snag a molecule passing by. After the protein binds to a molecule, the cell engulfs the part of its surface containing the protein, forming an internal bubble called an endosome. LRP2 then releases the molecule inside the cell, and the endosome carries the protein back to the surface.
To understand this shuttle system, Barasch and colleagues collected LRP2 from 500 mouse kidneys. The researchers put some of the protein in a solution at the extracellular pH of 7.5, and some in an endosome-mimicking solution at pH 5.2. Using a cryo-electron microscope, they captured images of the proteins and then stitched the images together in a computer, rendering 3-D maps of the protein at both open and closed formations.
The researchers suggest that charged calcium atoms hold the protein open at extracellular pH. But as pH drops due to hydrogen ions flowing into the endosome, the hydrogen ions displace the calcium ions, causing the protein to contract.
What's Your Reaction?


























































